GMP Certificate Attestation |Embassy Legalization
Good manufacturing practice/GMP Certificate is a documented proof that ensuring the products are consistently produced and controlled according to quality standards. When some manufacturing company wishes to export their products to abroad, then Good Manufacturing Practice / GMP Certificate Attestation from concern Embassy or consulate is required. To export some particular products, the GMP Certificate Attestation or Legalization is compulsorily required.
The Good Manufacturing Practice is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product. GMP Certification is a system to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process and also every time a product is made. Many countries have formulated their own requirements for GMP based on standard GMP.
A GMP Certificate is issued by a national or International regulatory authority of an exporting country. The Certification authority depends on the type of products are being manufacturing. The GMP Certificate confirms that the products are producing through industry standard process and quality. It gives an assurance that the products listed on the certificate are manufacturing through proper safety and hygiene.
Requirement of Good Manufacturing Practice/GMP Certificate Attestation
When an Indian manufacturing company starting the export business of a consumable products, then they have to complete the Good Manufacturing Practice Certificate / GMP Certificate Attestation from the concern Embassy or Consulate of Country present in India. The GMP Certificate Attestation is required for Custom Clearance purposes of the importing Country. There are couple of different types of agencies who provide GMP Certificate for Export.
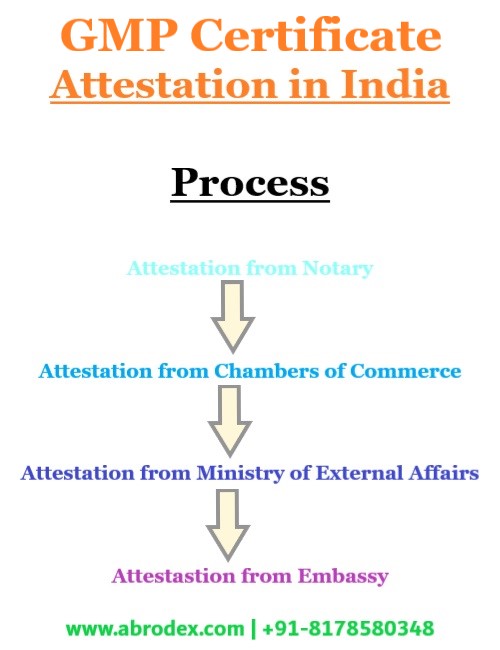
Process of GMP Certificate Attestation in India
The process of GMP Certificate Attestation in India is not a single step or direct process. To reach the final step of Attestation or Legalization, there are couple of necessary steps to go through. The GMP Certificate Attestation process starts with attestation from Notary and finishes with the Legalization by the Embassy or Consulate of the destination Country present in India.
Chamber of Commerce Attestation of GMP Certificate
A Businessmen association or network designed to promote and protect the interests of its members is generally called a ‘Chamber of Commerce’. It is also the organization for certification and it is the initial step for the commercial document Attestation. The Chambers of Commerce Attestation of GMP Certificate may be completed from anywhere in India or from that body where the company is registered.
MEA Attestation of GMP Certificate in India
Then Ministry of External Affairs Attestation i. e. MEA Attestation of GMP Certificate in India will be done after the Attestation from Chambers of Commerce. The MEA Attestation on GMP Certificate in India is the last stage of certification from the home Government. MEA Attestation of GMP Certificate Attestation in India may be completed from Delhi, Mumbai, Chennai, Kolkata, Hyderabad, Bangalore, Chandigarh, Guwahati etc. center.
Embassy Attestation or Legalization of GMP Certificate in India
When the GMP Certificate Attestation will be completed from Chambers of Commerce and Ministry of External Affairs (MEA), then the GMP Certificate Attestation from the concern Embassy or Consulate present in India will be done. It is the final stage of Attestation. It will be carried out by the officials of the destination Country’s Embassy or Consulate.
Time frame to complete GMP Certificate Attestation in India
The time frame to complete the GMP Certificate Attestation in India depends on various aspects. Normally, the whole process of GMP Certificate Attestation in India from Embassy will take about 8 to 10 working days. However, it can still stretch up to a couple of weeks more than a month due to lack of supporting documents.
Cost involved for GMP Certificate Attestation in India
The cost of GMP Certificate Attestation in India from Embassy in India varies from country to country. It might even be affected by the priority of the requirement. The Commercial Invoice Attestation cost may be higher or lower with the add-on facilities like pick and drop service of documents etc.
Attestation services are how someone can get suitable attestation for their commercial documents. Attestation agents at Abrodex Consultancy Services provides Attestation of the Certificate in India. Our all given Services are well-known and customer oriented. We do have good understanding of this field for about 10+ years and now we are swifter and quicker. We also offer pickup and deliver facility to suit your necessity.
We complete GMP Certificate Attestation and Legalization from the Embassy or Consulate of the following Countries present in India
